


Controllable. Versatile. Simple.
*
The IMPASS Embolic Device is an innovative deep, distally penetrating neurovascular embolic ideal for oncology uses as well as middle meningeal artery (MMA) embolization. It is designed for ease of use, versatility, and improved control during delivery.
-
No toxic and painful DMSO solvents
-
Simple preparation (ready-to-use syringe)
-
Radiopaque material allows for real-time visualization
-
Controllable material delivery
-
Consistent distal penetration without dilution
-
No risk of catheter entrapment
-
Deliverable through standard neurovascular microcatheters
-
No special delivery catheter or delivery systems required
-
Durable occlusion, independent of patient’s hemostatic condition
†
†
*
*
*
*
*
*
Limitations of Current Embolics
Embolic products available today can be difficult to use and unpredictable.
Microspheres & Particles
-
Off-Target Embolization
-
Proximal Reflux
-
Poor Visualization
-
Catheter Clogging
Coils
-
Inconsistent Embolization
-
Recanalization
-
Often require multiple coils to achieve desired occlusion (time, cost)
-
Risk of migration
Liquids
-
Special preparation & delivery technique
-
Toxicity of DMSO
-
Customization of NBCA
-
Catheter entrapment
-
Reflux, obstruction of catheter tip
Simple Preparation
No refrigeration required. Ready to use in under 30 seconds with no additional equipment or shaking.
Distal Penetration and Visibility
Pre-clinical delivery of IMPASS Embolic in an intercostal artery, simulating a middle meningeal artery (MMA) embolization.
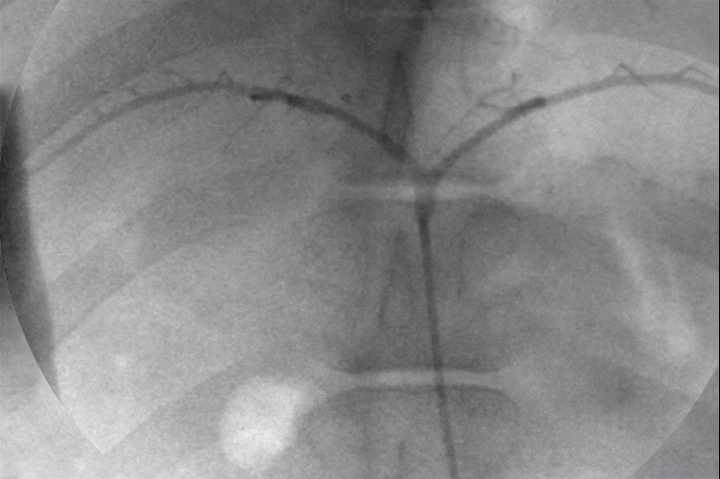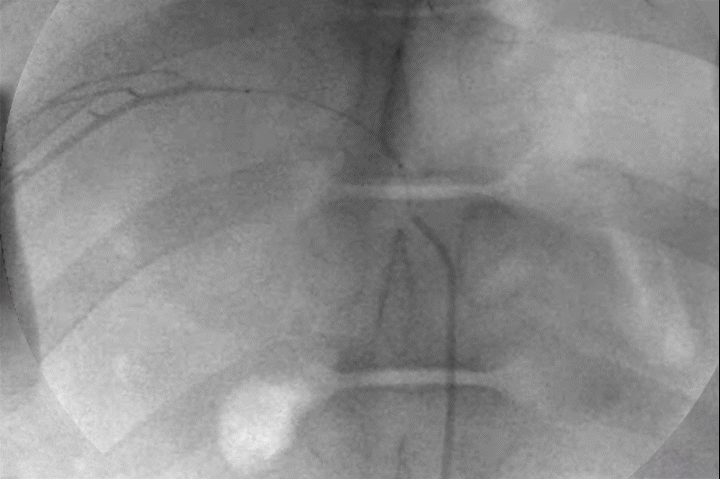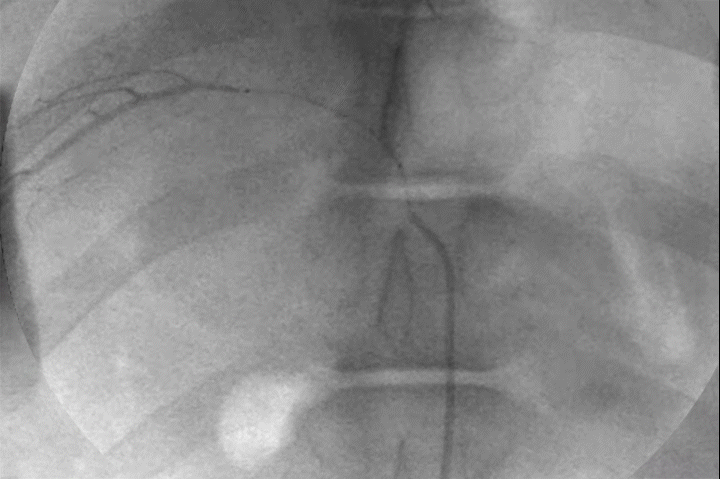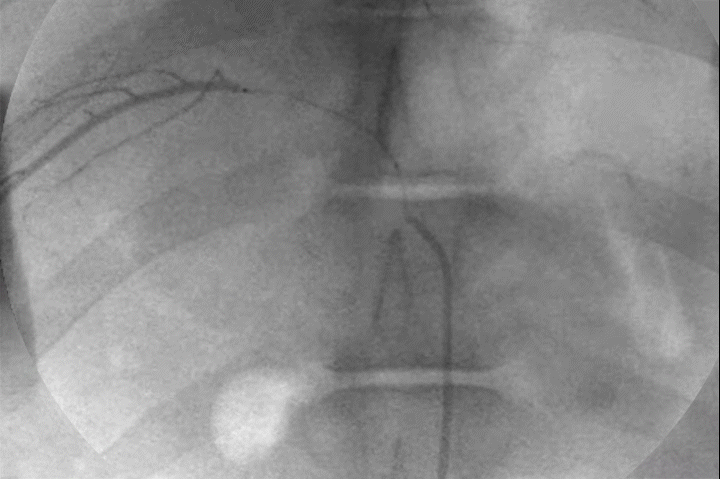
Mechanism of Action

-
The IMPASS Embolic Device contains a water-based ionic polymer solution in a delivery syringe
-
Counterions in the solution prevent polymer interactions in the syringe
-
When delivered to the bloodstream, the counterions immediately dissipate, allowing solidification of the polymer solution via ionic bonding
* Data on file. Fluidx Medical Technology, Inc.
† Based on in vivo study. Data on file. Fluidx Medical Technology, Inc. In vivo results are not necessarily indicative of clinical results.
The IMPASS Embolic Device is under development and does not have marketing clearance or approval in any market at this time.
Limitations of Current Embolics
Embolic products available today can be difficult to use and unpredictable.
Microspheres & Particles
-
Off-Target Embolization
-
Proximal Reflux
-
Poor Visualization
-
Catheter Clogging
Coils
-
Inconsistent Embolization
-
Recanalization
-
Often require multiple coils to achieve desired occlusion (time, cost)
-
Risk of migration
Liquids
-
Special preparation & delivery technique
-
Toxicity of DMSO
-
Customization of NBCA
-
Catheter entrapment
-
Reflux, obstruction of catheter tip
