

Controllable. Versatile. Simple.
*

*
The GPX Embolic Device is an innovative deep, distally penetrating peripheral embolic ideal for oncology and other devascularization uses. It is designed for ease of use, versatility, and improved control during delivery.
-
Simple preparation (ready-to-use syringe)
-
No special delivery catheter or delivery systems required
-
Controllable material delivery
-
Radiopaque material allows for real-time visualization
-
Durable occlusion, independent of patient’s hemostatic condition
-
No toxic and painful DMSO solvents
-
No risk of catheter entrapment
-
Deliverable through standard microcatheters
†
†
*
*
*
*
*
Limitations of Current Embolics
Embolic products available today can be difficult to use and unpredictable.
Microspheres & Particles
-
Off-Target Embolization
-
Proximal Reflux
-
Poor Visualization
-
Catheter Clogging
Coils
-
Inconsistent Embolization
-
Recanalization
-
Often require multiple coils to achieve desired occlusion (time, cost)
-
Risk of migration
Liquids
-
Special preparation & delivery technique
-
Toxicity of DMSO
-
Customization of NBCA
-
Catheter entrapment
-
Reflux, obstruction of catheter tip
Simple Preparation
No refrigeration required. Ready to use in under 30 seconds with no additional equipment or shaking.
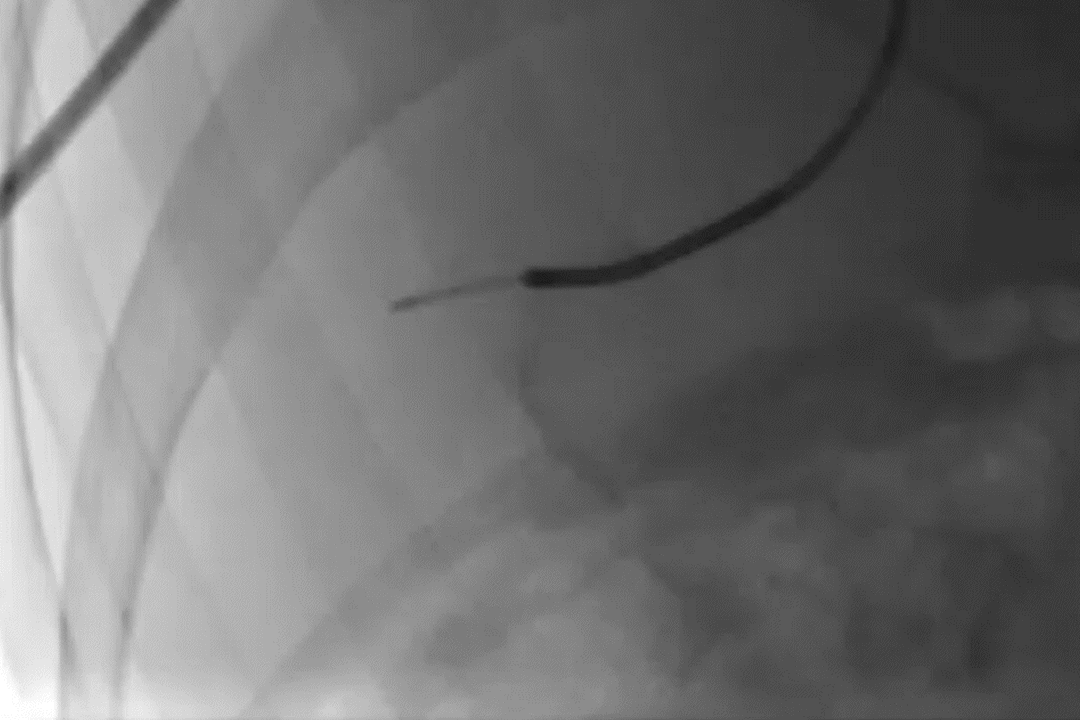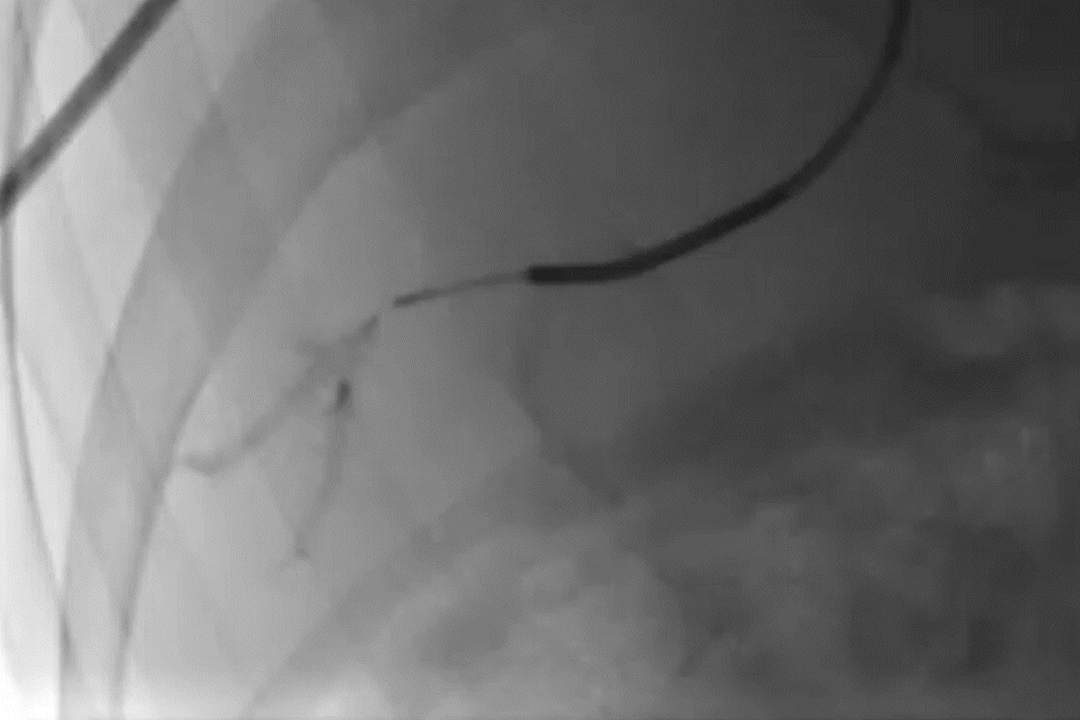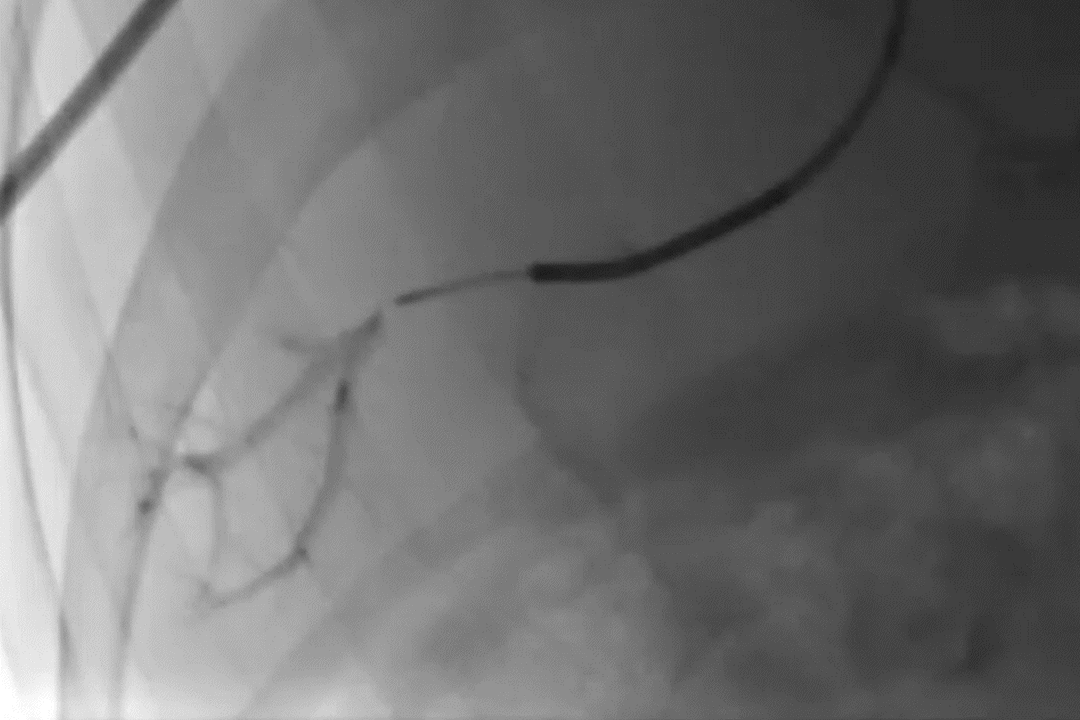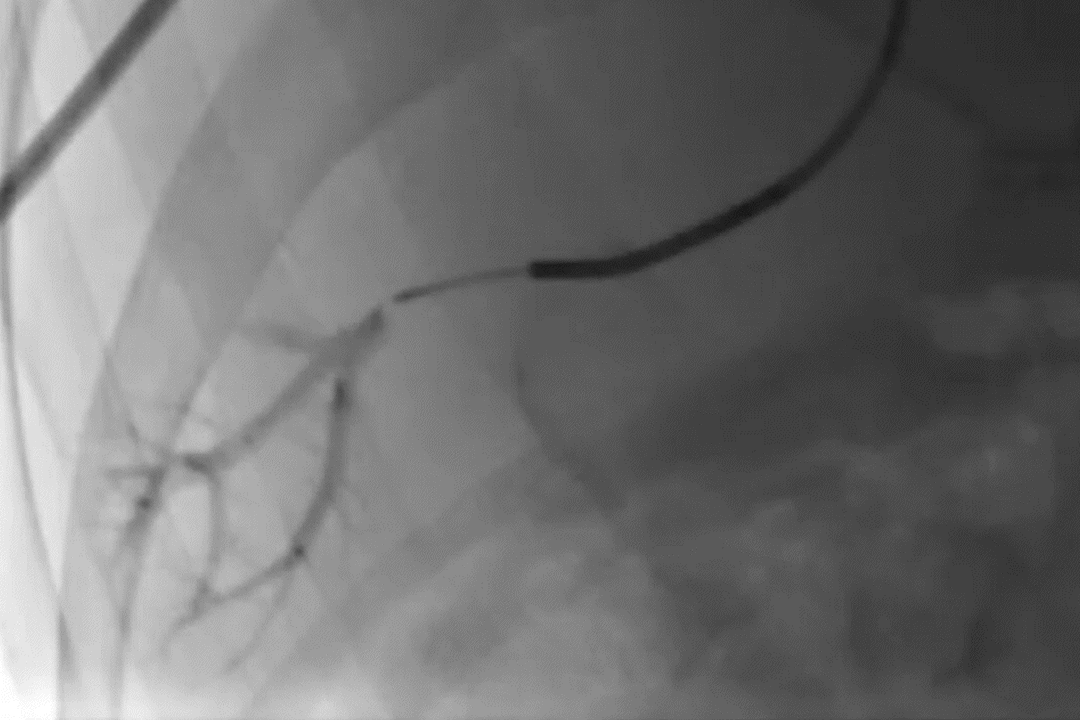
GPX Delivery to Portal Vein
GPX delivery to a portal vein demonstrates the distally flowing nature of the embolic.
Mechanism of Action

-
The GPX Embolic Device contains a water-based ionic polymer solution in a delivery syringe
-
Counterions in the solution prevent polymer interactions in the syringe
-
When delivered to the bloodstream, the counterions immediately dissipate, allowing solidification of the polymer solution via ionic bonding
* Data on file. Fluidx Medical Technology, Inc.
† Based on in vivo study. Data on file. Fluidx Medical Technology, Inc. In vivo results are not necessarily indicative of clinical results.
The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time.
Limitations of Current Embolics
Embolic products available today can be difficult to use and unpredictable.
Microspheres & Particles
-
Off-Target Embolization
-
Proximal Reflux
-
Poor Visualization
-
Catheter Clogging
Coils
-
Inconsistent Embolization
-
Recanalization
-
Often require multiple coils to achieve desired occlusion (time, cost)
-
Risk of migration
Liquids
-
Special preparation & delivery technique
-
Toxicity of DMSO
-
Customization of NBCA
-
Catheter entrapment
-
Reflux, obstruction of catheter tip
