


*
†
†
Controllable. Versatile. Simple.
*
GPX Embolic Device is an innovative embolic agent that is designed for ease of use, versatility, and improved control during delivery.
-
Simple preparation (ready-to-use syringe, no special materials or catheters required)
-
Controllable material delivery
-
Radiopaque material allows for real-time visualization
-
Occlusion is not based on a patient’s coagulation situation
-
Deep penetration into vessel beds
-
Durable occlusion

Clinical Need
Embolic devices are widely used to stop blood flow for targeted treatment of hypervascular tumors, vessel malformations, internal bleeds, and other cancer-related uses.
Some challenges that clinicians may experience with embolic devices today include:
Complicated and lengthy preparation process with special materials and delivery catheters often required
Lack of complete penetration of the vessel beds and tumor feeding vessels
Device control challenges and lack of real-time visibility during delivery which may result in less effective use and potential damage to healthy tissue
Reliance on a patient’s ability to coagulate blood flow, which can be limited in many very sick patients
Technology

Innovative Embolic Technology
*
-
Aqueous-based, low viscosity polymer solution in the delivery syringe
-
Ready-to-use, requires less than 1 minute of tableside preparation by the clinician
-
Can be delivered using standard microcatheters and no special solutions required
-
Solidifies rapidly upon delivery through an electrostatic mechanism of action without polymerization or dimethyl sulfoxide (DMSO) precipitation associated with other liquid embolics
-
Polymers bind upon delivery forming a durable, gel-like solid
Innovative GPX Embolic technology requires no DMSO and minimal preparation
GPX Delivery
Deployment of GPX in a portal vein segment
‡
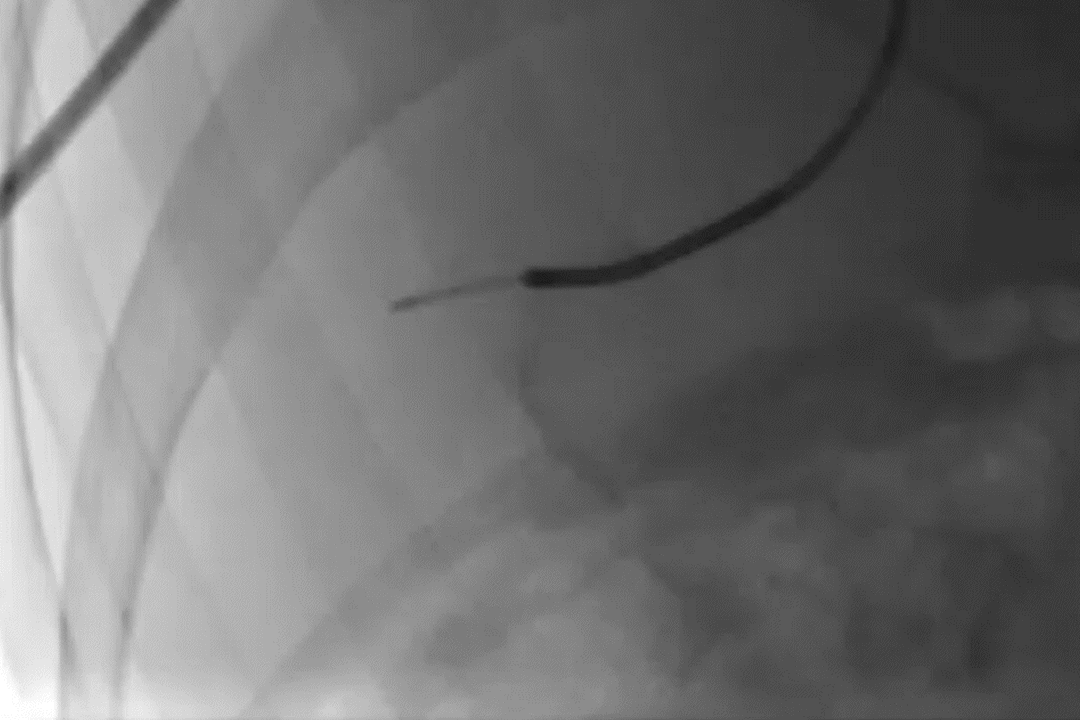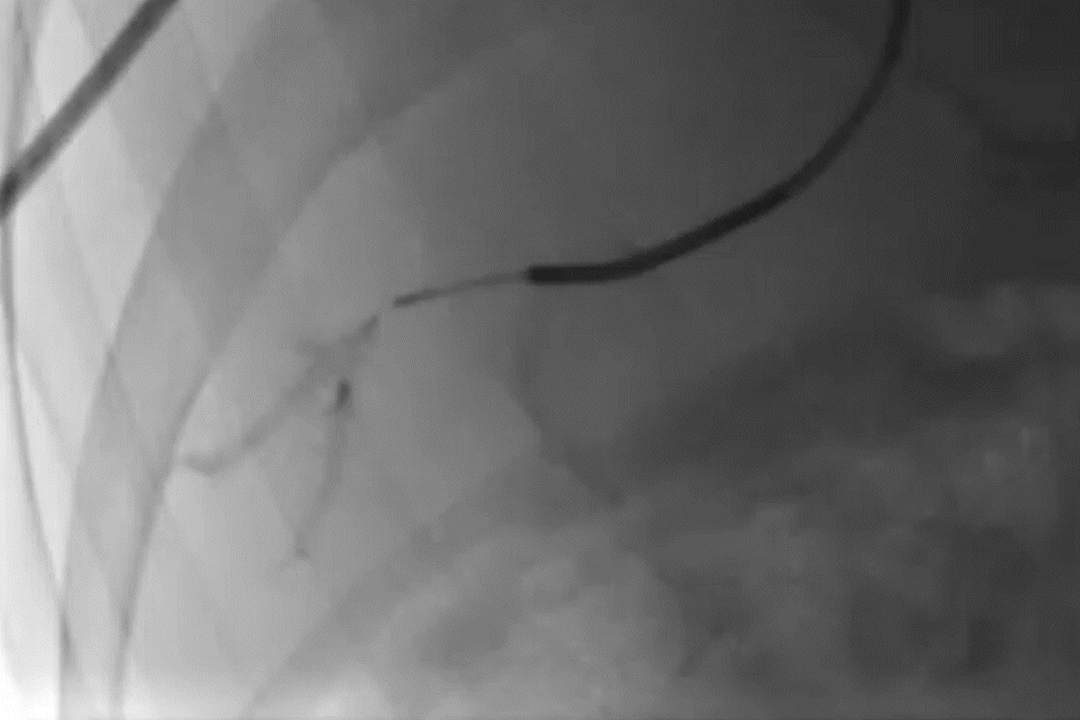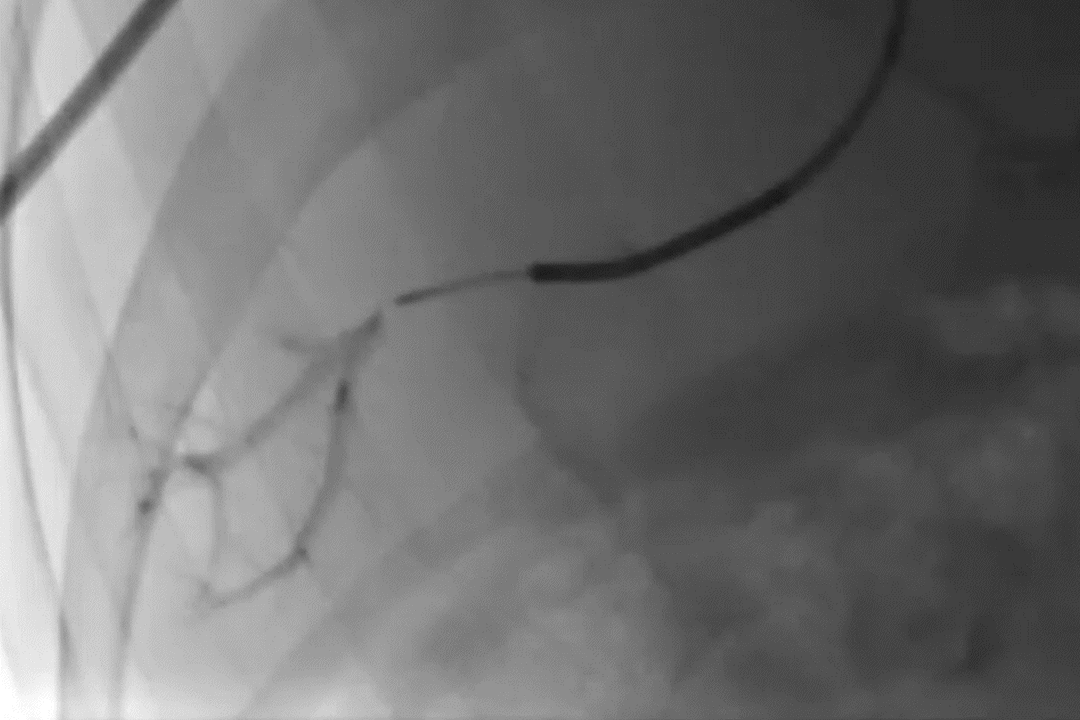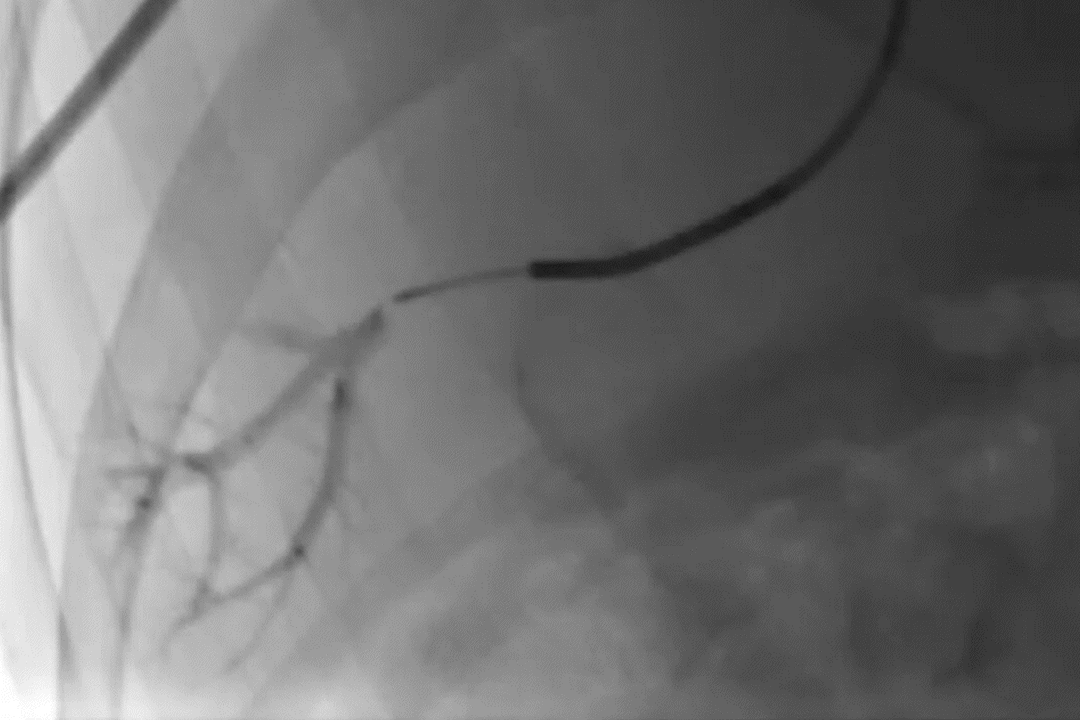
GPX-Clear Embolic Device*
The GPX-Clear Embolic Device leverages the core GPX technology as well as incorporates a non-artifact inducing radiopacity agent. The radiopacity agent then dissipates within 24 hours post-delivery, enabling unobstructed visibility of the treated area.

Pretreatment angiogram

Fluoroscopic image 1-day post-embolization showing dissipation of radiopacity

Fluoroscopic image taken immediately after delivery showing GPX-Clear Embolic Device placement

Post-treatment DSA showing complete occlusion of targeted region with GPX-Clear Embolic Device

Radiopacity 1-hr post-GPX-Clear Embolic Device delivery

Radiopacity dissipated at 24-hr post-GPX-Clear Embolic Device delivery
†
* Data on file. Fluidx Medical Technology.
† Based on in vivo study. Data on file. Fluidx Medical Technology. In vivo results are not necessarily indicative of clinical results.
The GPX Embolic Device is under development and does not have marketing clearance or approval in any market at this time. For investigational use (in New Zealand) only.
The GPX-Clear Embolic Device is under development and does not have marketing clearance or approval in any market at this time.
